Clinical Research’: The Pillar of Health-Care
A strong health care system is essentially like a boat that lets a nation sail through the sea of today’s dynamic world. It is no surprise hence that a sudden threat to healthcare can throw us all off-balance! This is evident with the COVID-19 pandemic that has shaken us in possibly every sector! ‘Clinical Research’, is an emerging and promising component of medical and health science, that intends to promote both the knowledge of human diseases and good health.
Clinical research provides a beam of hope for those willing to try new therapies and at the same time, opens tremendous career opportunities that accommodate people with a wide range of academic qualifications.
‘Clinical trials’ are an integral and valuable component of any research. They involve human subjects (healthy and diseased) to help determine the safety and effectiveness of medicines, healthcare devices, and diagnostic products. They also hold the key to unlocking the quick release of effective vaccines!
The sponsors or the contract research organizations (CROs) involved with the conducting of trials adhere to the guidelines of ICH (International Council for Harmonization) of Good Clinical Practice (GCP). The verification of patient consent, safety and integrity, data credibility, and outcome of trials would be verified by the ‘regulatory authorities.’
With the rapid pace of changing science, a single researcher cannot bring all the expertise to develop and validate innovations. Sharing of information between institutions is hence essential. Health information ‘databases’ are tailor-made to meet this requirement. ‘Databases are fast, economical, and help analyze large sets of data.
‘Clinical data management’ is one of the most essential sectors of clinical research. Databases that help detect unexpected or adverse events of drugs post-marketing, form the base of another major sector called ‘pharmacovigilance.’
Last but not least is the prime role of a ‘medical writer’, who develops the clinical trial documents that provide every detail of the trial along with the results or outcome.
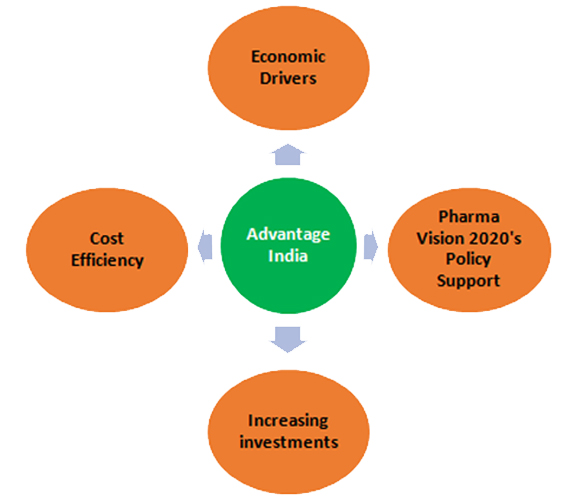
With India being the largest provider of generic drugs globally, it has an important position in the global pharmaceuticals sector and the market size is expected to only grow. Moreover, India’s prospects of being competent in clinical research that also goes hand-in-hand with pharmaceuticals is major because of proven success of outsourcing, large patient pool, availability of educated healthcare professionals, hospitals with good facilities, prevalence of diverse diseases and cost reduction. According to a source on Indian Pharmaceuticals Industry Report, the following factors put India at advantage:
With clinical research, there is hope for better medical care. Several diploma/certification courses in clinical research specifically train aspirants to mould them to suit such work environments. Vincent Van Gogh, the famous Dutch painter had once quoted, ‘Normality is a paved road: It’s comfortable to walk, but no flowers grow’. It is indeed true considering the present scenario where novel disciplines need a boost to overcome new challenges such as the prevailing pandemic!
If you found this article informative. Share it on your Facebook profile, Linkedin, Twitter, Instagram.
Comments
Post a Comment